Page 32 - SaxoCell Annual Report22/23
P. 32
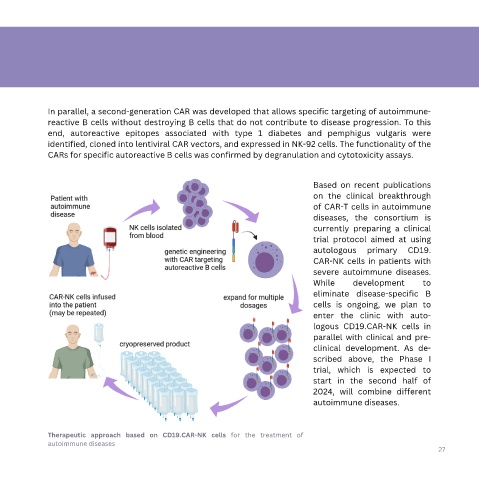
In parallel, a second-generation CAR was developed that allows specific targeting of autoimmune-
reactive B cells without destroying B cells that do not contribute to disease progression. To this
end, autoreactive epitopes associated with type 1 diabetes and pemphigus vulgaris were
identified, cloned into lentiviral CAR vectors, and expressed in NK-92 cells. The functionality of the
CARs for specific autoreactive B cells was confirmed by degranulation and cytotoxicity assays.
Based on recent publications
on the clinical breakthrough
of CAR-T cells in autoimmune
diseases, the consortium is
currently preparing a clinical
trial protocol aimed at using
autologous primary CD19.
CAR-NK cells in patients with
severe autoimmune diseases.
While development to
eliminate disease-specific B
cells is ongoing, we plan to
enter the clinic with auto-
logous CD19.CAR-NK cells in
parallel with clinical and pre-
clinical development. As de-
scribed above, the Phase I
trial, which is expected to
start in the second half of
2024, will combine different
autoimmune diseases.
Therapeutic approach based on CD19.CAR-NK cells for the treatment of
autoimmune diseases
27